How I Discovered Gamma Delta T Cells that Help Cure Cancer
I decided to contribute this article after reading the ESRA journal article by Dr. Adler of a medical "first": the heart transplant in Gruute Schur Hospital, Cape Town. My father, Harry Bank, trained at Gruute Schur before coming to Israel with his equally Zionist wife, Myra, as Mahal volunteers for the War of Independence. The education Ronnie, my brother, and I received growing up in north Tel Aviv, which included reading books such as Microbe Hunters by Paul de Kruif and the example set by my physician-dad`s tendency to think "out of the box" in medicine, instilled in me an urge to try and discover something new and meaningful in science when I grew up. This is a concise report of how this dream was accomplished. And that "something" is now known as the Gamma Delta T cell.
A bit of background first. The immune system of our body constantly monitors for antigens, which are hallmarks of infectious agents or cancer. Antigens are recognized as foreign to us by "antibodies" and by "T cells" which together activate a complex response to remove them and restore health.
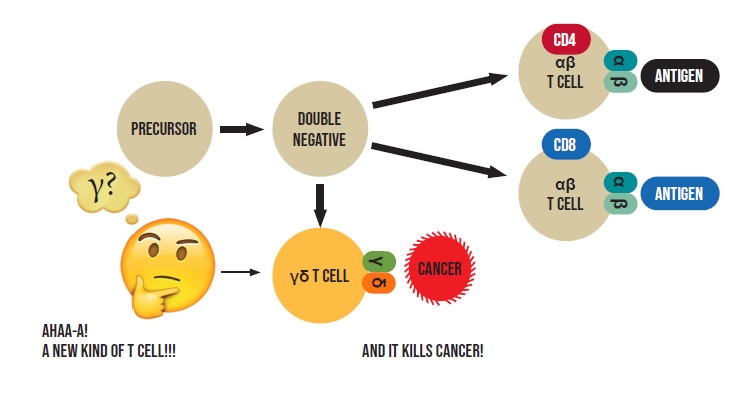
Antibodies are soluble proteins secreted by plasma cells, and were well defined by the 1950s and 60s. The critical issue of how T cells detect foreign antigens, was more complex and remained enigmatic until the mid-1980s when it was at last determined that T cells use a "T cell receptor" encoded by two genes, named alpha (a) and beta (b), together creating the "ab T cell receptor" to recognize foreign antigens and initiate complex mechanisms to get rid of the invading "bad guy" - whether a virus, bacterium or a cancerous cell. T cells acquire an "ab" T cell receptor when they develop in an organ called the thymus, after having matured enough to previously express one of two additional molecules - called CD4 and CD8 (Figure 1).
I arrived in Dr. Len Chess` laboratory at Columbia University in New York in 1982 after completing my military service as a physician in the IDF. My project was to help determine the specific functions of CD4 and CD8 in T cells by isolating T cells from the human thymus (the organ where T cells develop) that had not yet matured enough from precursor cells, to express CD4 or CD8 (called double negative T cells) (Figure 1). We did this by removing from the thymus all cells that expressed CD4 or CD8 then expanding, in the lab, the vanishingly small number of remaining double negative T cells to obtain enough "double negative" cells to use in future experiments. To our surprise, we found that in contrast to the prevailing dogma that only mature T cells, that had already expressed CD4 or CD8, could express a T cell receptor, our cultured double negative T cells exhibited features strongly suggesting they were also expressing a T cell receptor. However, we determined that the putative receptor we were observing was definitely not an ab T cell receptor. We were perplexed.
Then, one day, Len rushed into the lab brandishing a paper that had just been published in the leading scientific journal, Nature. It showed that a recently discovered new "mysterious" gene named gamma (g) of unknown function but bearing similarities to the known a and b T cell receptor genes, was highly expressed in "double negative" thymocytes of mice. Despite its resemblance to the a and b T cell receptor genes investigators had not been able to show that it participated in creating a T cell receptor and were stumped as to its function.
Having already made the discovery that our "double negative" thymocytes, appeared to be expressing a T cell receptor, albeit distinct from the previously described ab T cell receptor, we quickly tested the possibility that our putative new receptor was instead encoded by the "mysterious" g gene. Indeed, we next demonstrated that g was highly expressed in our double negative thymocytes and along with another gene, which was later named d, participated in forming a previously unpredicted T cell receptor in "double negative" T cells (Figure 1). Moreover, I showed that when stimulated appropriately, this new T cell receptor could trigger the "double negative" T cells to kill cancer cells! (Figure 1).
Our discovery, which was supported by independent findings by scientists from the Dana Farber cancer institute at Harvard University, led to recognition of the existence of an entirely new and previously unanticipated class of T cells, which came to be known as "gd T cells" (Figure 1). The scientific paper describing our findings, and that of the Harvard groups were published jointly in Nature in 1986. They were recognized in 2016, 30 years later, by the American Association of Immunologists (AAI) as "Pillars of Immunology" and reprinted in their official journal.
>12,000 scientific papers later, the critical role of this new kind of T cell in regulating multiple aspects of our normal health has become abundantly clear. Moreover, the principle of maintaining three types of antigens recognizing molecules (antibodies, ab T cell receptors, and gd T cell receptors) has been preserved in all vertebrates during their evolution over 400,000,000 years. So, from a bench in a relatively small laboratory, our research, and that of other groups of scientists had culminated in the unveiling of a third form of an immune receptor, 400 million years after its emergence in evolution.
While many aspects of the gd T cells are still enigmatic, they clearly play a unique and very important role in processes such as infections, cancer, and metabolism, as partly anticipated by our early finding that, in the test tube, these cells could very efficiently recognize and kill cancer cells. The efforts of many scientists have led, over the past few years, to the establishment of at least 10 biotechnology companies worldwide, with investments exceeding 300 million dollars, which are exploring ways to use them to cure cancer, and very promising results are anticipated in the coming year. Most exciting is the report when on December 6 , 2021 a company named Adicet revealed data from its first clinical study. They showed that gd T cells that were harvested from healthy people and modified appropriately in the laboratory, then infused into patients with end stage cancer of the lymph (lymphoma) (in whom all previous forms of very advanced therapy had failed), completely eradicated the cancer in three of four of the patients in this initial study.
My father, Harry Bank, who was a great clinician, was impressed by my research efforts, but was anxious to see how they could be applied clinically. It took more than 30 years to finally come to fruition. As of now, it appears that my discovery of gd T cells, a fulfillment of a childhood dream, has led to a meaningful breakthrough in clinical medicine as well. This latest kind of T cell, when modified appropriately, appears to eradicate advanced cancer in patients, and its application will probably affect medical conduct in meaningful ways in the coming decades.It is a message of great hope for patients with hitherto incurable diseases.







Comments